RESEARCH
-

GOAL
The main goal of our research is to understand how brains learn and store information, from the molecular level through in vivo neuronal networks and how these processes go awry in neurological disorders. We are focused on elucidating the gene program that is induced by learning and required for long-term forms of brain plasticity and memory consolidation.
-

APPROACH
We employ an interdisciplinary and collaborative approach that intersects diverse fields such as virology, evolutionary biology, biochemistry, and neuroscience. Our unique toolkit spans protein biochemistry to in vivo manipulation of neuronal circuits involved in behavior.
-

QUESTIONS
What is the role of intercellular Arc communication in memory formation? What cargo is transported by Arc capsids and extracellular vesicles? Does intercellular Arc play a role in neurodegenerative diseases? Is Arc unique, are there other proteins that can form capsids? How do synapses and epigenetic processes mediate plasticity and memory?

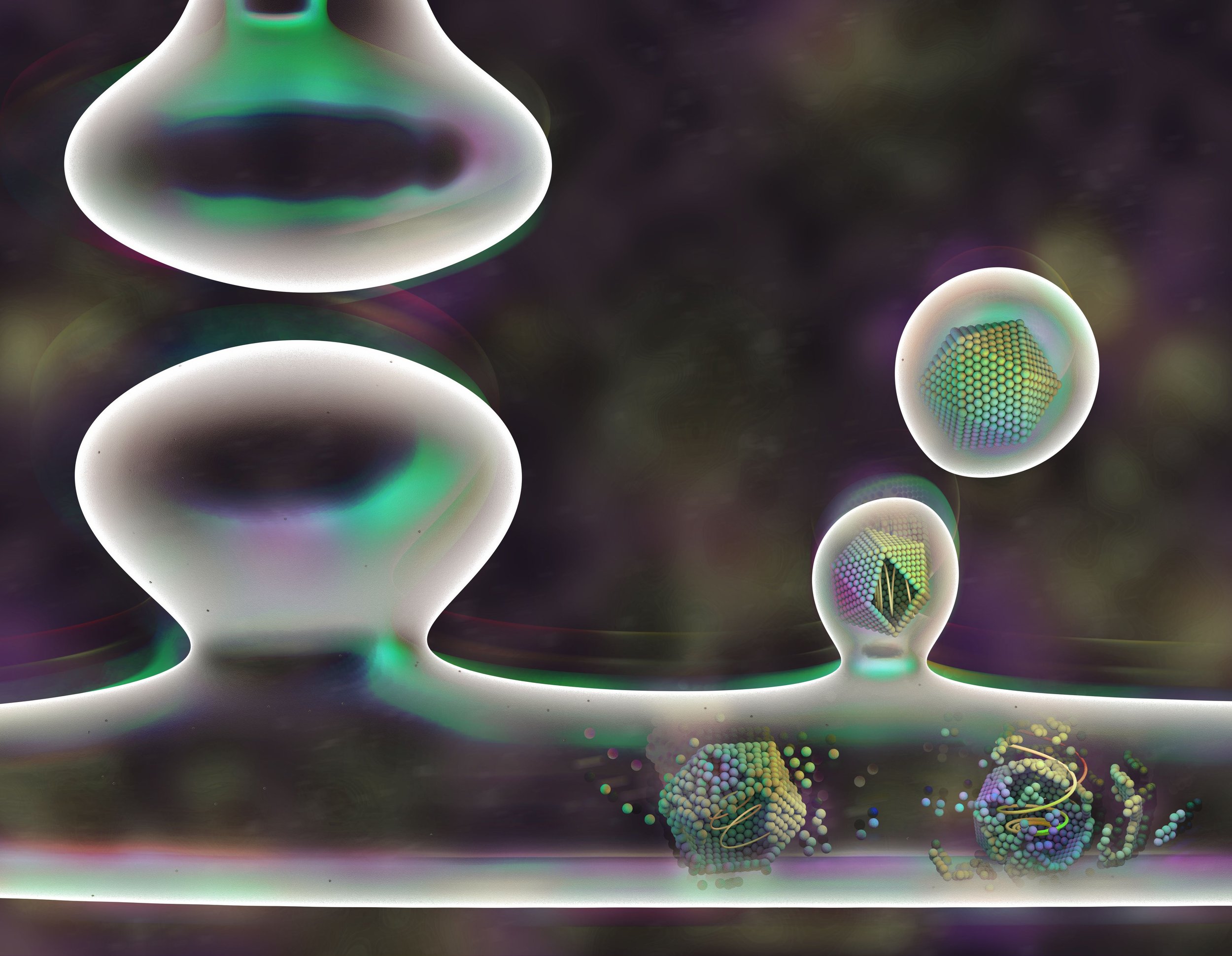
DISCOVERY
We recently made the surprising discovery that the neuronal gene Arc shares biochemical properties with retroviruses; Arc protein can form virus-like capsids that encapsulate RNA that are released from cells in extracellular vesicles.
CURRENT RESEARCH PROJECTS
-
We recently discovered a novel mechanism of neuronal communication that resembles the life-cycle of retroviruses. We found that Arc contains a Gag retroviral homology domain that has conserved secondary structure with retroviruses like HIV-1, which evolved from a distinct family of retrotransposons. Arc protein self-assembles into viral-like capsids that are released from cells via extracellular vesicles and carry RNA/proteins to neighboring cells.
There are a number of fundamental cell biological questions that need to be addressed: What cargo does Arc transfer cell-to-cell? Which cells take up Arc vesicles? Where and when does Arc form capsids in neurons? Moreover, there are dozens of putative animal genes that contain similar Gag-like domains that could confer related viral properties. The ability to form capsids and transfer genetic material may not be unique to Arc. Thus, our work may have uncovered a new mode of cellular communication derived from the repurposing of retroviral biology. The lab’s future work will shed light on the evolutionary origins of cognition and the cell biology underlying information storage in the brain.
Neurodegenerative diseases are commonly caused by pathology or dysfunction in a focal part of the brain. However, over time, pathology spreads to other regions. In the case of Alzheimer’s disease, pathology usually starts in the temporal lobe and spreads to connected brain regions. Little is known about how pathology spreads. We hypothesize that extracellular vesicles may mediate the spread of pathology and are currently investigating the role of Arc in these processes.
-
Cortical circuits that process visual information require normal visual input during critical windows of development. Abnormal visual input during development leads to impaired vision, such as Amblyopia. Failure to correct Amblyopia early in development can lead to permanent impairment of vision that cannot be corrected in adulthood. Current treatments block input (monocular deprivation, MD) to the good eye, to “train” the amblyopic eye. This drives plasticity in the primary visual cortex (V1), which ultimately results in weakening input from the good eye and strengthening input from the weak eye. However, failure to achieve normal vision with this treatment can occur because plasticity in the visual cortex diminishes after a ‘critical period’. The major goal of this project is to determine novel ways to enhance plasticity in V1.
In animal models of MD, an ocular dominance (OD) shift in V1 occurs where the deprived eye first becomes weaker and then the open eye stronger. OD shifts are driven by NMDA-type glutamate receptor (NMDARs) dependent synaptic plasticity, through long-term potentiation (LTP) and long-term depression (LTD). We found that Arc is essential for normal OD plasticity and trafficking of AMPA-type glutamate receptor (AMPAR) trafficking in V1. We also recently found that overexpressing Arc protein can enhance OD plasticity in adult V1 and can regulate the development of binocular vision. Strikingly, manipulating Arc acutely in adult V1 can rapidly alter binocular visual response properties.
Goals of this project are: 1. Identifying the molecular mechanisms of Arc plasticity in vivo. 2. Understanding how Arc expression is regulated and why it declines during development. We are directly investigating these aspects of V1 plasticity using the latest in vivo imaging techniques and new molecular reporters of synaptic function developed in the lab. -
Synaptic plasticity and memory involve spatiotemporal fine-tuning of gene expression levels in response to environmental stimuli, including rapid transcription of immediate early genes on the time scale of minutes and longer-term global chromatin remodeling. We are investigating the epigenetic elements that govern activity-dependent expression of key genes that underlie learning and memory (https://www.nature.com/articles/s41593-020-0634-6). Key questions include how the 3D genome is modified during learning and how these modifications impact memory consolidation.
-
The overarching goal of this project is to characterize the protein machinery that mediates trafficking of postsynaptic membrane proteins critical for synaptic plasticity. Endo- and exocytosis of membrane proteins are critical trafficking steps in all cells, but are especially dynamic and finely tuned in neurons. Unlike presynaptic endocytosis and synaptic vesicle recycling, the precise molecular processes that underlie postsynaptic trafficking still remain poorly defined. Little is known about how specific receptors are removed from the protein scaffolding complex at the postsynaptic density (PSD) or the functional role of protein-protein interactions within the PSD during synaptic plasticity. Our previous work showed that Arc regulates AMPA-type glutamate receptor trafficking (https://www.sciencedirect.com/science/article/pii/S0896627306006829 and https://www.sciencedirect.com/science/article/pii/S0896627306006830). We conducted unbiased proteomic screens and discovered novel Arc interacting proteins. We are investigating how these proteins help Arc recruit AMPA receptors out of the post-synaptic density. To study protein trafficking within nanodomains of synapses we have developed a novel live light microscopy approach. These studies will provide mechanistic insight into how Arc mediates multiple forms of synaptic plasticity and also broadly elucidates the protein machinery that is involved in postsynaptic trafficking of membrane proteins at excitatory synapses.
